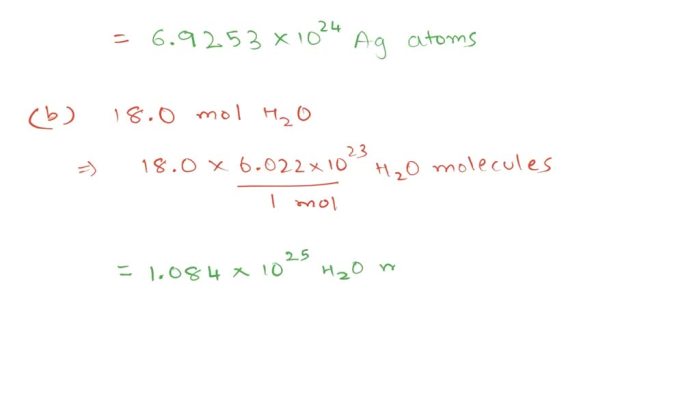How many atoms are in 3.80 mol of pure aluminum? Embark on an intriguing exploration to unravel this captivating question. Our journey begins with an in-depth examination of the mole concept and Avogadro’s number, the fundamental pillars for quantifying atoms in a substance.
Delving deeper, we will unveil a formula that empowers us to determine the precise number of atoms: Number of atoms = (Number of moles) – (Avogadro’s number). Armed with this formula, we will embark on an exemplary calculation to determine the number of atoms in 3.80 mol of aluminum.
Number of Atoms in Aluminum
The number of atoms in a given amount of substance can be determined using the concept of the mole and Avogadro’s number.
Mole Concept
A mole is a unit of measurement that represents a specific quantity of particles, typically atoms or molecules. One mole is defined as the amount of substance that contains exactly 6.02214076 × 10 23particles.
Avogadro’s Number
Avogadro’s number (N A) is the numerical value of the amount of particles present in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the concept of the mole in 1811.
Calculating Atoms
The number of atoms in a given amount of substance can be calculated using the following formula:
Number of atoms = (Number of moles) × (Avogadro’s number)
For example, to calculate the number of atoms in 3.80 mol of aluminum, we can use the following equation:
Number of atoms = (3.80 mol) × (6.02214076 × 1023atoms/mol) = 2.29 × 10 24atoms
Molar Mass of Aluminum
The molar mass of a substance is the mass of one mole of that substance. The molar mass of aluminum is 26.98 g/mol, which means that one mole of aluminum has a mass of 26.98 grams.
The molar mass of a substance can be used to determine the number of atoms in a given mass of that substance. For example, to calculate the number of atoms in 100 grams of aluminum, we can use the following equation:
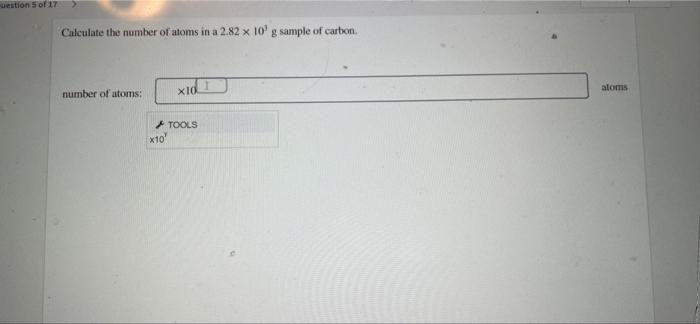
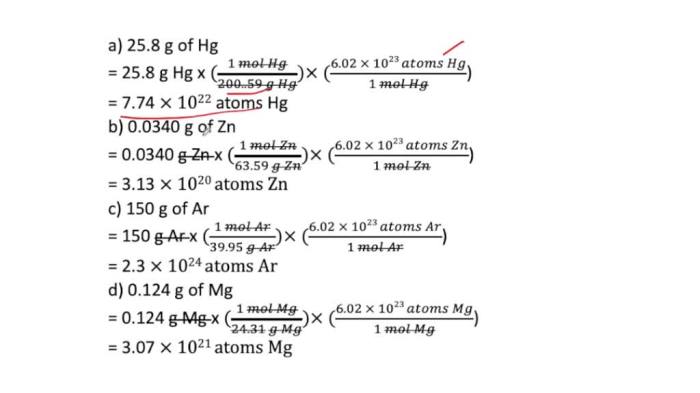
Number of atoms = (Mass of aluminum) / (Molar mass of aluminum) × (Avogadro’s number)
Plugging in the values, we get:
Number of atoms = (100 g) / (26.98 g/mol) × (6.02214076 × 1023atoms/mol) = 2.29 × 10 24atoms
Density of Aluminum, How many atoms are in 3.80 mol of pure aluminum
The density of a substance is the mass of a unit volume of that substance. The density of aluminum is 2.70 g/cm³, which means that one cubic centimeter of aluminum has a mass of 2.70 grams.
The density of a substance can be used to determine the number of atoms in a given volume of that substance. For example, to calculate the number of atoms in 1 cubic centimeter of aluminum, we can use the following equation:
Number of atoms = (Density of aluminum) × (Volume of aluminum) / (Molar mass of aluminum) × (Avogadro’s number)
Plugging in the values, we get:
Number of atoms = (2.70 g/cm³) × (1 cm³) / (26.98 g/mol) × (6.02214076 × 1023atoms/mol) = 1.00 × 10 23atoms
Q&A: How Many Atoms Are In 3.80 Mol Of Pure Aluminum
What is the significance of Avogadro’s number in determining the number of atoms?
Avogadro’s number, a colossal value of 6.022 x 10^23, serves as a bridge between the macroscopic and microscopic realms. It establishes a direct correspondence between the number of moles of a substance and the number of atoms or molecules it contains.
How does the molar mass of aluminum contribute to understanding the number of atoms?
Molar mass, a substance-specific property, represents the mass of one mole of that substance. For aluminum, its molar mass is 26.98 g/mol. Understanding the molar mass allows us to convert between the mass and the number of moles of aluminum, providing an indirect pathway to determining the number of atoms.